If you’re a skincare nerd, you may know that different skincare oils have different fatty acid profiles – that is, they differ in terms of which fatty acids they contain e.g. linoleic, linolenic, oleic and lauric acid.
The fatty acids have interesting properties. For example, lauric acid is strongly antibacterial, and works better than benzoyl peroxide against Cutibacterium acnes bacteria. Oleic acid is a barrier disruptor, while linoleic acid can fade pigment. So it sounds like skincare oils could be interesting active ingredients or irritants, alongside moisturisation!
Related post: Do oils make your skin less oily? The myth of rebound oil
But there’s a catch: in oils, these fatty acids aren’t actually acids at all. They’ve undergone a chemical reaction to form new substances called triglycerides. These triglycerides can be broken down to form the free fatty acids again under the right conditions: during digestion, and in the process of saponification in soap-making. But does this happen on the surface of your skin?
I’ve been wondering about this for a while, and I’ve had a few chats on and off with fellow nerd Stephen of Kind of Stephen over the years but we never really came to any firm conclusions. I finally sat down recently and dug up all of the papers I could find on fatty acids and combed through them all to try to make some sense of it (there were literally about 400 papers that I sorted through, which is why I couldn’t bring myself to do it earlier… I probably didn’t need to go through so many, but I like to be extra thorough to convince myself thrice over before trying to convince anyone else!).
A disclaimer: I don’t recommend trying to sift through the literature to “discover” new things in research-heavy areas (e.g. acne and other skin disorders) because rest assured, researchers who know a lot more about this stuff and have a ton more training have already done this and published it in a review, and if you manage to find something new it’s probably not supported by the research.
But whether skincare oils break down into free fatty acids is not the sort of topic that gets funded, so I feel safe(ish) making my own conclusion here, although I welcome review!
Related post: Are unsaturated oils bad for your skin?
Here’s the video on YouTube, in which I go through the chemistry of fatty acids and triglycerides, the key studies, and explain the (sort of, kind of, tentatively convincing) conclusion I came to: triglycerides in oils are not broken down to give significant amounts of free fatty acids on the skin’s surface. In other words, we can’t rely on oils for the properties of their free fatty acids except when the FFAs are effective at very low concentrations.
Keep scrolling for the text version…
Are Oils Equivalent to Fatty Acids?
Do oils convert to fatty acids on your skin? This question might sound really abstract and theoretical, but it’s fundamental to understanding why some skincare claims about oils aren’t as valid as they sound.
For example, you might have seen that lauric acid is 15 times better at killing acne bacteria than benzoyl peroxide. Coconut oil is 50% lauric acid. Does this mean that coconut oil is going to be fantastic at killing acne bacteria?
Or: oleic acid disrupts your skin barrier. Argan oil contains about 50% oleic acid. Will argan oil disrupt your skin?
What is a fatty acid?
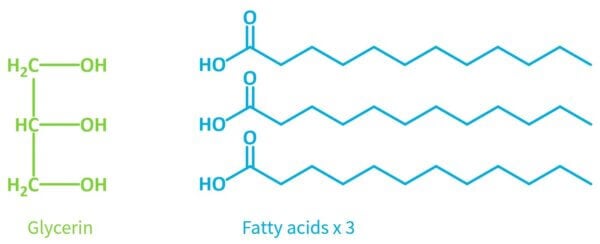
To work out what’s going on, let’s start by talking about the difference between fatty acids and triglycerides.
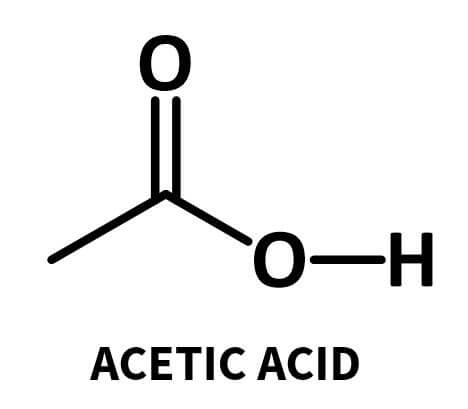
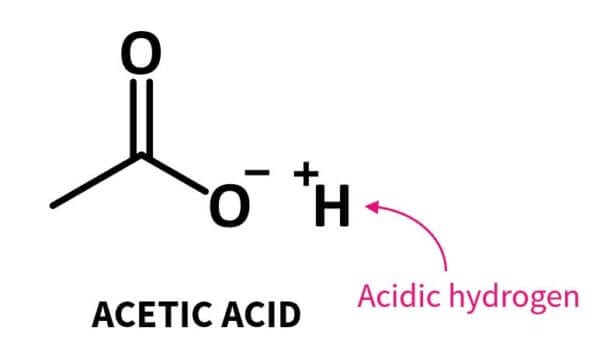
An acid in organic chemistry is a group that contains a carbon double bonded to an oxygen atom and an OH group:
The hydrogen atom can ionise (break off and become a H+ ion), which is what makes it an acid.
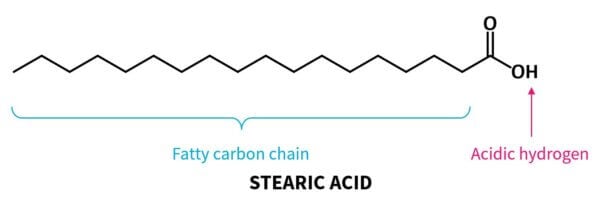
Fatty acids have this acid group connected to a long non-polar chain made of hydrogen and carbon atoms. This chain makes the chemical feel greasy and fatty, hence the name “fatty acid”.
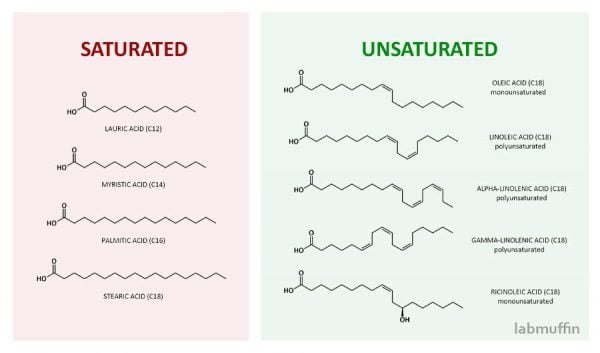
There are a range of fatty acids:
- Saturated fatty acids contain no double bonds e.g. lauric, myristic, stearic and palmitic acids
- Monounsaturated fatty acids have one double bond e.g. oleic and sapienic acids
- Polyunsaturated fatty acids have multiple double bonds e.g. linoleic and linolenic acids
Some unsaturated fatty acids are called omega fatty acids (“omega” refers to where the double bonds are).
What is a triglyceride?
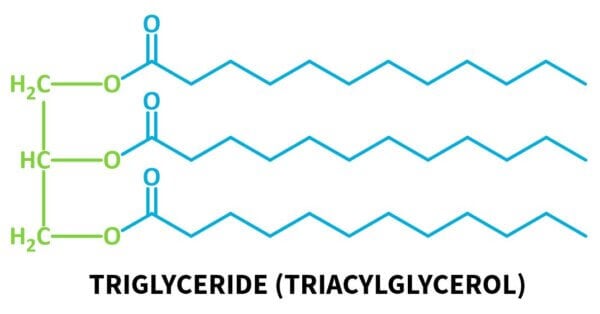
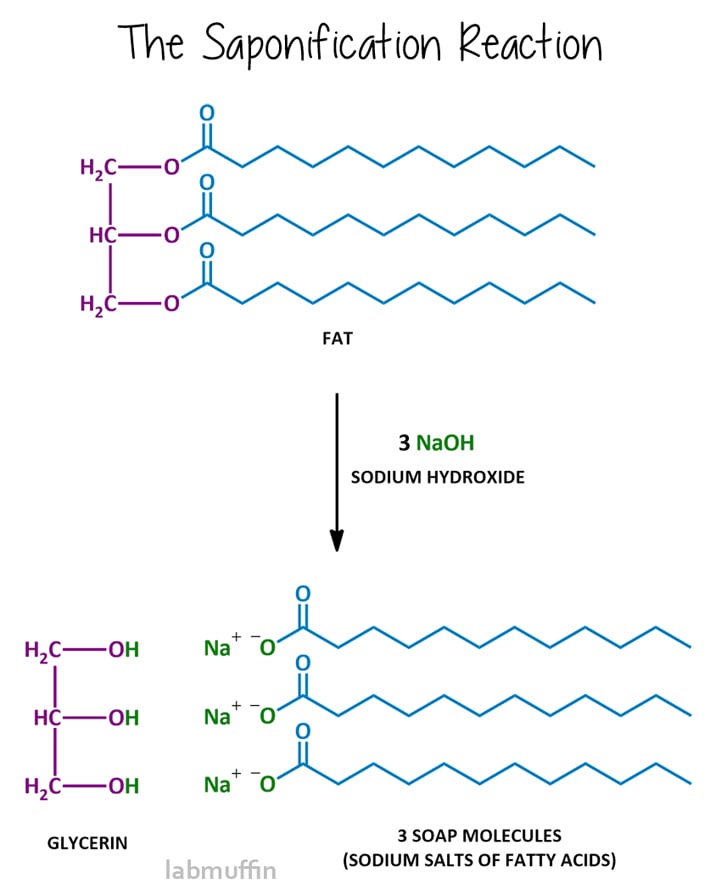
A triglyceride (also called a triacylglycerol) looks like this:
It has one glycerin molecule connected to three fatty acids via ester linkages.
Triglycerides are the main components of the common fats and oils that you’ll know, like the vast majority of plant oils (olive oil, sunflower oil, coconut oil), but also fats like beef fat and lard.
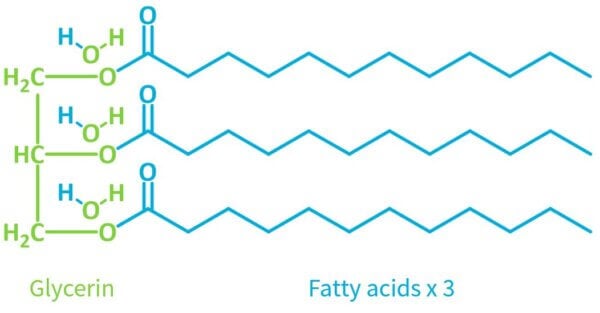
If you zoom into the ester linkages in a triglyceride, you’ll notice that the COOH acid groups aren’t there anymore. There technically aren’t any fatty acids in the triglyceride at all!
That’s because the acids have undergone a chemical reaction to make the triglyceride, so they’re no longer acids. The OH on the fatty acid and a H on the glycerin both comes off, and they connect:
So you can see the acid no longer has that hydrogen atom that can come off, so it’s no longer an acid.
It’s a little bit like how sodium metal and green chlorine gas undergo a chemical reaction to give sodium chloride, which is white and salt and delicious, not green or metallic or gas-like at all.
But chemical reactions can be reversed under the right conditions. For triglycerides, there are two common situations:
Nutrition
You’ll see lots of nutritional advice saying that fish oil is a good source of essential omega-3 fatty acids, even though the actual fish oil contains only triglycerides. Lipase enzymes from your saliva, stomach and pancreas break up the triglycerides and turn them back into free fatty acids. This breakdown reaction is called hydrolysis.
Soap-making
Soap makers also talk about oils in terms of the percentages of fatty acids contained in them, because the main step in soap making involves performing a chemical reaction with lye to break up the covalent bonds in the triglycerides to free the fatty acids.
What about in skincare?
When we put plant oils on our faces, we’re applying very little free fatty acids. In general you’ll have less than 1% free fatty acids in the oil that haven’t been either transformed into the triglyceride, or have broken off the triglyceride.
Too many free fatty acids actually makes the oil rancid which is when it smells and tastes off. For example, coconut oil can’t be sold if it contains more than 0.5% free fatty acids. Virgin coconut oil has an average of 0.1% free fatty acids. Cold-pressed unrefined rose hip oil has about 2% free fatty acids.
So the question is: do we have lipases on the surface of our skin that can break up these triglycerides?
If yes, then using oils is the same as using the free fatty acids.
But if not, then it’s like going to a restaurant and ordering a steak and being given the steak while it’s still attached to the cow.
It turns out that the answer is quite complicated.
I’ve included the most relevant references here, but if you’re really interested in the science and the chemistry behind it all I’d encourage you to do a proper literature review yourself.
Lipases in the skin
There are enzymes called lipases in your skin that can break up triglycerides. Some of these are made by humans but a ton of these are made by microorganisms living in your skin.
Most of these live under the surface of your skin in your pores. Humans produce an oil called sebum in the sebaceous glands under the skin. When it’s at the bottom of the pore near the sebaceous gland it contains a high amount of triglycerides and almost no free fatty acids, but by the time it gets to the skin’s surface around 1/4 of this has broken up to give 15% free fatty acids.
Scientists in the 70s did some experiments and found that P acnes, the acne bacteria, is the main microbe that produces these lipase enzymes that break down the triglycerides, but other species can produce lipase enzymes too. For example Malassezia fungus, staph and corynebacterium.
The vast majority of these organisms and their lipase enzymes sit inside the pores. There’s also a lipase that your body makes in the living epidermis of your skin that then gets released into the stratum corneum with the rest of the lipids.
What do the studies say?
So there are lipases and theoretically they can break down the triglycerides to give free fatty acids – but how much and how fast?
There aren’t that many studies that we can look at to see how much and how fast triglycerides get broken down to give the free fatty acids when they’re applied to the surface of your skin, so here’s what I’ve managed to find.
Tracing triglycerides breaking down on skin
In the 1950s, scientists applied radioactive tripalmitin (the triglyceride of palmitic acid) to someone’s back for 2.5 hours, then wiped it off and analysed it. 6% of the radioactive tripalmitin was broken up into free fatty acids. For comparison, around 30% of the triglycerides in regular sebum are broken up.
So why was this figure so low? The scientists suggested that the oil on the surface couldn’t get into the pore where the lipase enzymes were. And even if it did, it would probably get pushed out by new sebum that was coming out of the sebaceous glands.
6% in 2.5 hours sounds like quite a lot, especially when you think about how long oil stays on your skin overnight. But only a really tiny amount of the tripalmitin was used in this experiment – 0.5 milligrams or 0.56 microlitres, which is about one hundredth of a drop, applied over 300 square centimetres. If you use more oil, then it’s even less likely that it’ll get into the pore and so you’ll have an even lower percentage broken up into free fatty acids.
Impacts of triglycerides vs free fatty acids on skin
Another study looked at mixtures of free fatty acid oleic acid, and glycerol trioleate (the triglyceride with 3 oleic acid molecules bonded to glycerin).
Oleic acid as a free fatty acid is known to be a barrier disruptor. They made a bunch of mixtures:
- 0% free fatty acid + 100% triglyceride
- 25% free fatty acid + 75% triglyceride
- 50% free fatty acid + 50% triglyceride
- 100% free fatty acid + 0% triglyceride
They applied one drop of each mixture to the forearms of 12 people, covered it with gauze, gave them plastic wrap so they could cover it while they were showering, then looked at the sites the next day.
First they measured the transepidermal water loss (TEWL), the leakiness of the skin to water leaving. They found that the sites with 100% triglyceride had no change from the untreated areas of skin.
The TEWL for the other sites was directly proportional to the amount of free oleic acid. The scientists also measured how easily chemicals could penetrate the skin going in using fluorescein dye. The depth of the penetration of the dye was proportional to the amount of oleic acid as well.
So these results suggest that only tiny, negligible amounts of triglyceride were actually broken up into the free oleic acid. Or at least, any free oleic acid that formed was immediately turned into something else that didn’t disrupt the skin barrier, or the free fatty acids didn’t end up in the same place (i.e. on the skin).
With any of these explanations, it’s clear that applying free oleic acid and applying oleic acid bound up in triglycerides did not have the same effect, at least within the time frame of the experiment (24 hours).
Other forms of fatty acids in skin
There was also a third study, which looked at what happened when triglycerides were applied to skin over a longer period of time.
The scientists made a cream containing linoleic acid-rich sunflower and borage seed oils, along with a plain placebo cream. Both creams were applied twice daily to the dry inside forearm skin of 11 volunteers during winter. Each person applied the active cream to one arm, and the plain placebo cream to the other arm.
The scientists then analysed the lipids in the stratum corneum, in terms of the ceramide, free fatty acid and cholesterol content. After 4 weeks, there were actually less free fatty acids in the area treated with oil cream compared to plain cream. However, the levels of ceramide 1 linoleate (ceramides with linoleic acid attached) were nearly doubled on the oiled part compared to the placebo treated skin, while ceramide 1 oleate (same ceramide with oleic acid attached) was much lower.
Interestingly, the sebum’s free fatty acid portion actually has less unsaturated acids like linoleic acid and oleic acid than in the original triglycerides.
This suggests that some free fatty acids (linoleic acid and oleic acid) are selectively removed after triglycerides are hydrolysed or broken down, and used to make other substances like ceramides.
So what does this mean?
From these studies, we can see that triglycerides applied on skin aren’t broken down to give you free fatty acids on your skin – in other words, applying oils isn’t the same as applying the free fatty acids in those oils.
This is important because in pharmacology the dose is very important. For example, one ibuprofen tablet is less effective than two. One ibuprofen tablet might get rid of your headache, but taking tiny portions of one tablet over three days won’t be noticeable.
It’s even more complicated because breaking down the triglycerides depends on microorganisms, and different people have different microorganisms on their skin. For example, men have more microbes on their skin than women. Other factors like climate and genetics will make a big difference too.
Different parts of your face and body also have different microorganisms. One study found that sebum triglycerides were hydrolysed more quickly at the nose than on the forehead.
Taking antibiotics will change your skin’s microbes, but so will other things like using different cleansers, creams and antibacterial products.
So in conclusion we shouldn’t rely on oils for the properties of their free fatty acids, unless they’ve been shown to be effective at very low concentrations . Even then, there’s a caveat: having the fatty acids in an oil and having them in a solution of alcohol (how they usually test the activity of these free fatty acids) will have very different effects on how well they penetrate your skin.
But this doesn’t mean that all oils are useless! They can still be very beneficial.
For example, oils are often excellent moisturisers. They have an occlusive effect and slow down how quickly water leaves your skin, increase hydration. They also work as emollients that soften your skin.
Oils are also very good bases for oil soluble ingredients. Sometimes, they naturally have beneficial ingredients in them. For example, rosehip oil contains a few forms of vitamin A – mostly beta carotene, but there’s also a tiny amount of retinoic acid (tretinoin) in there as well. They can also potentially be good sources of fatty acids for making ceramides in your skin, as shown in the study on sunflower and borage seed oils.
Verdict
Oils contain fatty acids bound up in triglycerides, not free fatty acids. Applying skin care oils won’t give you the same effect as applying the free fatty acids.
Studies on Oil Hydrolysis to Free Fatty Acids on the Skin
Key Studies
The studies I talk about in depth:
Nicolaides N, Wells GC, On the biogenesis of the free fatty acids in human skin surface fat (open access), J Invest Dermatol 1957, 29, 423–433.
Mack Correa, MC et al., Molecular interactions of plant oil components with stratum corneum lipids correlate with clinical measures of skin barrier function (open access), Exp Dermatol 2014, 23, 39–44.
Conti A et al., Seasonal influences on stratum corneum ceramide 1 fatty acids and the influence of topical essential fatty acids, Int J Cosmet Sci 1996, 18, 1–12. DOI: 10.1111/j.1467-2494.1996.tb00131.x
Other relevant studies
Some of the other relevant papers that I came across in my search:
Shalita AR, Genesis of free fatty acids (open access), J Invest Dermatol 1974, 62, 332–335.
- Review of experimental evidence for bacterial lipases hydrolysing triglycerides in sebum – the key species is P acnes
Anderson RL et al., Individual and site variation in composition of facial surface lipids (open access), J Invest Dermatol 1972, 58, 369–372.
- Triglyceride hydrolysis happens faster in nose follicles than the nose surface than the forehead surface
- There was a lot of variation between individuals
- Less oleic and linoleic acid in sebum at surface compared to in triglycerides at sebaceous gland
Bomar L et al., Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols (open access), mBio 2016, 7, e01725-15.
- Study on bacteria in nose that hydrolyse triglycerides, and discusses how colonisation varies between different people
Fierer N et al., The influence of sex, handedness, and washing on the diversity of hand surface bacteria (open access), Proc Natl Acad Sci USA 2008, 105, 17994–17999.
- Gender differences in bacteria on skin surface
Hall GS, Mackintosh CA, Hoffman PN, The dispersal of bacteria and skin scales from the body after showering and after application of a skin lotion (open access), J Hyg 1986, 97, 289–298.
- Impact of skincare products on skin surface bacteria
And in case you’re wondering, my favourite skincare oils are rosehip oil, hempseed oil and sunflower oil.
Related posts: Why Linoleic Acid and Rosehip Oil Might Fix Your Skin, Review of Oils for Oil Cleansing
This post contains affiliate links – if you decide to click through and support Lab Muffin financially (at no extra cost to you), thank you! For more information, see Disclosure Policy.





Hi Michelle,
Thanks for this incredible article.
Have you heard about Korean cosmetic ingredient company Labio’s fermented oil series? Supposedly the process increases the amount of free fatty acids. I can see how this (might?) be desirable if targeting for linoleic acid but get this…
Most ingredients in the series have an olive oil base with, according to their own literature, dramatically increased free oleic acid. Huh?
Their promotional material: http://labio.kr/?lang=US&tpf=board/view&board_code=4&code=35
Confused,
Elise
Hmmm that sounds weird! Free oleic acid doesn’t sound like a good thing…
So oils containing oleic acid are safe to use? I was so worried because I’m an oil junkie and very obsessive I was so close to throwing out everything conating oleic acid! Does the same go for polyunsaturated oils?
Hi Michelle,
This is an amazing post, thank you so much! This debunks (or implies debunking) of so much shoddy skincare quasi-science.
Quick questions – must a triglyceride always be composed of the same three fatty acids, or can it be mixed? From the last study, it appears as if triglycerides could be used to change the balance of ceramides in the skin – is there a desirable balance?
Thank you!
Really interesting and informative read/video!!!
One thing that I’ve always wondered about is how much of the skincare is actually absorbed and how much evaporates when we apply it. I have the feeling that most of it actually evaporates and it doesn’t sink in…
It depends on the product – most things don’t evaporate (their molecular weights are too high)!
This is why I could never attempt to be a proper INCI blogger: I would feel the need to go through all the papers myself, and I just can’t be bothered to do that along the reads I need to do for my job that is not beauty related. So thank you for taking the time and presentin your conclusion here.
This is THE best video explaining different fatty acid profiles! Thanks so much!
So glad I came across this video <3
Yay! I’m so glad you liked it 🙂
Excellent video Michelle
Is there any enzymes that is skin safe and be added in skincare products to release FFA.
Not as far as I know!
Brilliant as always, Michelle! I have a question. The studies that showed high linoleic sunflower oil helped restore barrier function, and high oleic olive oil reduced barrier function…if the fatty acids aren’t free, what could the likely mechanisms of action be here?
The triglycerides would be hydrolysed after absorption into the skin and immediately converted to something else, so there’s no significant amount of free fatty acids anywhere.
That is very interesting indeed. The question has crossed my mind, but I wasn’t invested enough to do a literature research and definitely not invested enough to go through 400 papers! Thank you for offering the breakdown.
Thank you Anne!
There are new studies about to see how much and how fast triglycerides get broken down
Would you be able to link the ones you’re referring to please? Thank you!
Thank you for all the research you put into this and for sharing! I love learning about the plant oils 😊 I have been researching oils and butters for a while now and testing different ones on my skin for 30 days at a time to experience their different qualities and to see what kind of effect using each one has on my skin. Properties such as absorption speed, viscosity, etc. we often read are based on the fatty acid profile. For example the semi solid oils are said to have this solid at room temp trait because of high levels of saturated fatty acids. But, if I’ve understood the info you have presented correctly, then these types of traits may have been misattributed to the fatty acids? 🤔 I would love to hear if you have any insight into this.
The physical properties usually come from the saturated carbon chains, which align and form crystalline structures more readily – the saturated carbon chains will be there in the free fatty acids, and are still there when the fatty acids are bound up in triglycerides, so the properties carry through regardless of whether they’re free or not. Hope that makes sense!
This was amazing- thank you!!- But because I am not a chemist, just a beauty industry pro- I don’t fully understand the conclusion. I am an oil junkie and love using oils such as Rosehip, avocado, squalene, rice bran etc.. – am I doing harm to my skin? help!
(btw- thank you for taking the time to research this, I have gone down the rabbit hole of other cosmeceuticals in an effort to better understand ingredients that are truly active- and whoa! It sure is a never-ending rabbit hole of information)- so I “get” that you have invested extraordinary effort into this- THANK YOU
If you like them that’s great! It’s just that you’re probably not going to get the pigment fading etc. properties of linoleic acid, just the properties of the oil (mostly moisturising).
Do you think that rosehip oil is still worth using if it’s mainly for moisturizing properties only? Or would it be better to just save money and use Vaseline/mineral oil since they are more moisturizing anyway? I was interested in rosehip oil for the antioxidant aspects, but now I’m not sure if it’s worth the cost.
LOVE your blog. And. Still so grateful for you taking the time to do written articles with your movies. I much prefer learning by reading.
Great article! What is your take on Ethyl Linoleate? According to raw material supplier: it is a more stable form of linoleic acid which “gets converted to linoleic acid in-vivo and provides all the skin benefits of linoleic acid with improved skin permeability”? Thank you!
It sounds like it could potentially work quite well! Do they have any evidence to show the conversion and clinical effects?
Thanks for reply! The supplier did in vivo clinical study that showed statistically significant improvement in skin texture, hydration, tone, firmness & radiance, which all sounds great. I wasn’t able to find any studies showing the conversion from ethyl linoleate to linoleic acid in vivo. I’m going to ask the supplier for this documentation.
Michelle, hello! Thank you for your blog, it is very interesting and useful. The other day I came across a study of oils as enhancers and their effect of natural oils on the fatty acid content of the epidermis and dermis. The study was conducted ex vivo in 2017. It is freely available. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. If you are familiar with this study, you could tell why the fatty acid composition of the skin changed when different oils were applied to it. Can the conclusions of this study be trusted? Sincerely, Victoria.
The study assumes that it’s just the free fatty acid contaminants in the oils having the effect, and there’s no contribution from the triglycerides e.g. “Natural oils are heterogeneous lipid mixtures composed of triglycerides and minor components—mono- and diglycerides, free fatty acids (FAs), phosphatides, sterols, fatty alcohols, fat-soluble vitamins, and other substances [4]. FAs are the main components in natural oils which can modify the skin barrier.”
“It must be noted that predominant components in natural oils are triglycerides while FAs are minor constituents along with mono- and diglycerides, phosphatides, sterols, fatty alcohols, fat-soluble vitamins, and other substances” (whereas if they also counted the fatty acids bound up in triglycerides, the fatty acids would be the major constituent)