Since writing this post on the influence of pH on exfoliating acid skincare ingredients, I’ve had a lot of questions on how much active free acid there is in specific formulations. The calculation is a bit fiddly to do every time, so I’ve developed an easy spreadsheet to help you predict the free acid content for a product, if you know the amount of the acid ingredient in it, and its pH. This might also be useful if you’re into making your own acid products, or messing around with the strengths of pre-made products.
Refresher on Acids and pH
pH is a measure of how acidic or basic something is – a lower pH is more acidic, which means there’s a higher concentration of H+ ions.
AHAs and BHAs (alpha and beta hydroxy acids) are common exfoliating ingredients. They’re acids, which means they can exist in two forms:
- as the free acid, which means it has no charge
- as the ionised or dissociated form, which means it’s lost a H+ and it has a negative charge (this is also called its conjugate base)
The two forms can interconvert. Here’s what they look like for glycolic acid:
The ionised form has a difficult time getting through skin’s oily lipid layer and into the skin where it can act. The free acid form is a lot more oil-soluble, so it penetrates a lot more easily. (In some circumstances, if the ionised acid is non-polar enough, it can still penetrate through skin.)
As pH decreases, more of the free acid form will be present, so more exfoliant will be absorbed through the skin. At high pH, more of the ionised form will be present, so it won’t get through skin as well. Hence low pH is better for exfoliation, but it also means there will be more irritation. Different acids will have different proportions of the free acid and ionised forms at different pHs, reflected in a property called pKa.
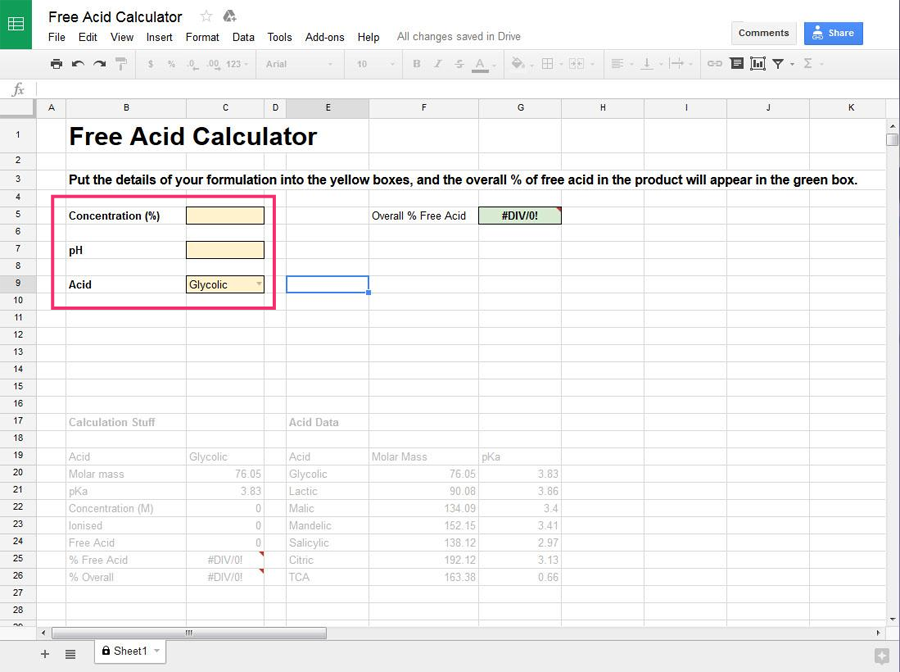
How to Use the Free Acid Calculator
You need to know:
- the concentration of the acid ingredient in your product (either in the product information, or you can ask the manufacturer)
- the pH of the final product (again, you can ask the manufacturer or test your product using pH strips or a pH meter)
- the name of the acid (straight from the ingredients list)
Enter this information into the yellow boxes on the left of the sheet (select the name of the acid from the dropdown menu).
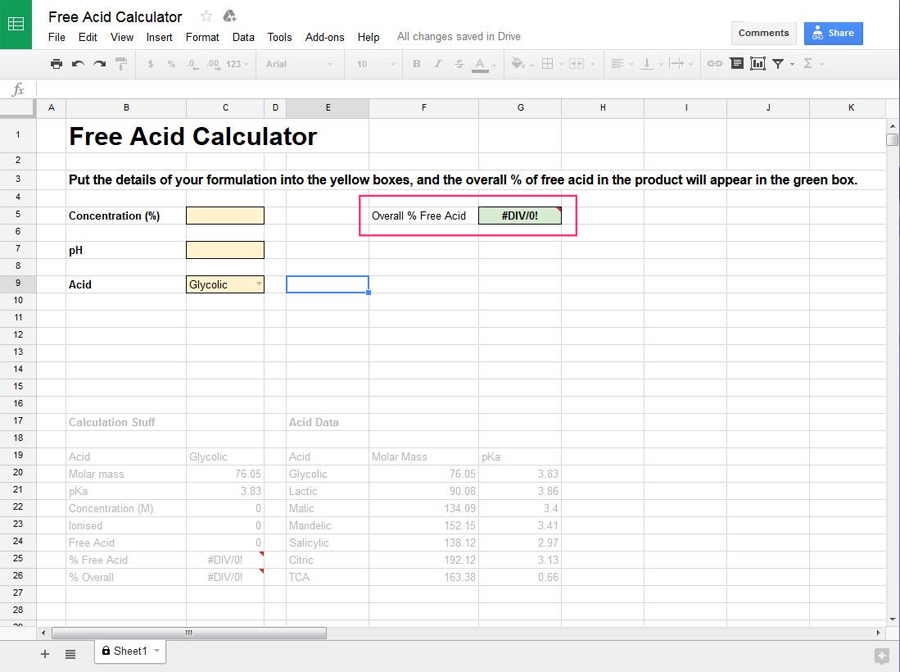
Once you’ve entered the information, the overall percentage of free acid in the product will be calculated and show up in the green box on the right.
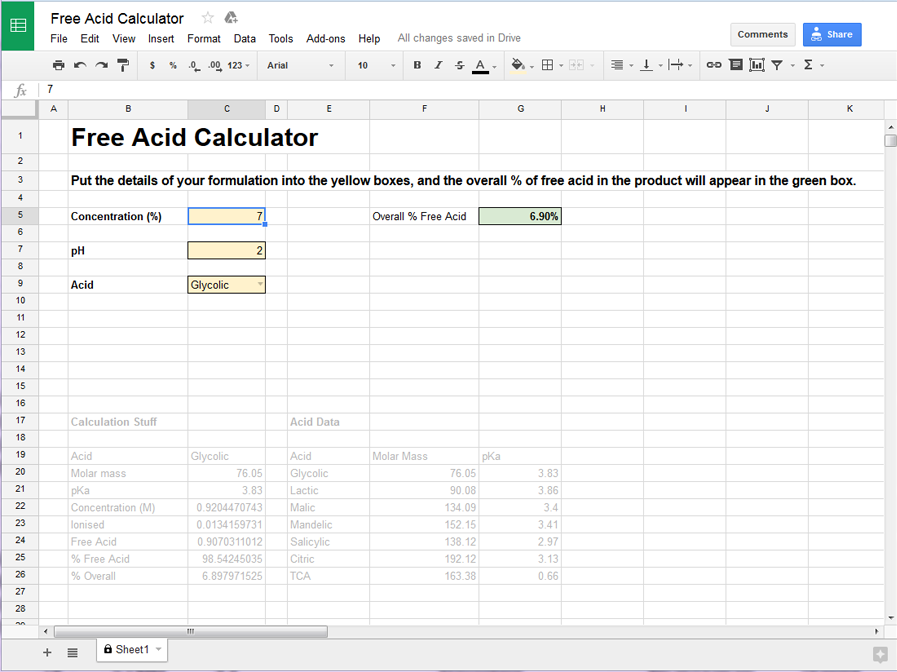
Here’s how it looks for a product containing 7% glycolic acid, at a pH of 2 – the calculated overall free acid content is 6.9%.
Easy! The free acid calculator has data for calculating the free acid content for the following acids:
- Glycolic
- Lactic
- Malic
- Mandelic
- Salicylic
- Citric
- TCA
- Ascorbic
If you’d like me to add more acids to the calculator, please let me know in the comments!
VERY IMPORTANT NOTE: There isn’t much point in using this to work out what a product can do for you – the free acid value isn’t going to translate into specific skincare effects! Pharmacology isn’t that straightforward. Everything else in the product – the solvent, the emulsifier – and how you use the product and how much you use is going to have a big effect. There’s also the fact that a product with a higher amount of total acid with the same free acid value as a product with a lower amount of total acid will probably have a depot effect, which will change how it’ll work on your skin. I go into this in more detail in this video.
The Mathematics Behind the Free Acid Calculator
Here are the nitty gritty details behind the calculations if you’re interested. It’s a variation of the Ka calculation that’s taught in first year university chemistry. If you see any errors, please let me know! You can see the formulae used in the spreadsheet if you click on the boxes. The greyed out area underneath has the juicy bits, and the pKa values and molar masses of each acid are shown on the right (they’re sourced from Wikipedia).
Assumptions:
- Product is primarily aqueous
- Product has a density of around 1 g mL-1
- Further dissociations are negligible
P.S. If this is all a bit too advanced, you can get the lowdown on all the basics of exfoliation in my free downloadable guide – find it here!









You’re so resourceful! Thank you so much for sharing, and giving me more motivation to keep learning about cosmetic chemistry!
Thank you! I’m glad you like it 🙂
This is awesome thanks!!
Wow! This is awesome. Now we will be aware of how many ph level a product has. Thanks for bringing it up!
Thank you so much for this!
You are amazng!!
Thank you! *blushes*
I agree. You are amazing.
I am not amazing or smart. Could you please answer a dopey question for me. I have a cream that has ammonium lactate. That means it’s already buffered, right? Will it break down into free acid? I think it’s 12%, with pH between 4.5and 5.5 (that’s what the PI says).
Would it be better to just get a Ponds formulation that has just plain lactic acid listed as an ingredient?
Ammonium lactate is made up of ammonium and lactate ions, so you can treat it a bit like lactic acid – you have to do a small conversion though! 12% ammonium lactate translates to 10% lactic acid, then you can plug it into the calculator with the pH.
Thank you SO, SO much. How can afford to be SO generous with your time?!
Again, thank you.
xoxo
Very helpful post, but what about betaine salicylate? Thank you!
It’s pretty much the same as salicylic acid, but at a lower concentration (assuming it dissociates from the betaine in solution). It’s roughly half, so for example if you have 4% betaine salicylate you should be able to use the calculator for salicylic acid at 2%.
Hello, could you explain in depth how your calculator works?
As in how to use it, or what the calculations behind it are?
Which is the pH of salicylic acid 2% ?
It can be anything… the pH can be adjusted (and is almost always adjusted) before the formulation is finished.
The free acid calculator is read only. Is it possible to get access to the calculator? I am trying to determine a concentration and pH of salicylic acid that will exfoliate well and still be tolerable.
I was using lactic acid in combination but it seems it may be too strong in free acid content at a pH of 3.3-3.5
Thanks for any input
Try making a copy and saving it to your drive – in the “File” menu, click on “Make a Copy”.
Can you add values for lactobionic acid and gluconolactone?
Thank you 🙂
If I bought a high-percentage salicylic acid product with a pH of 6, and simply add enough citric acid to lower the pH to around 3.5 or 4, will I still get the benefits of the salicylic acid? Or does salicylic acid always have to be kept at the appropriately low pH at all stages of manufacturing for it to be effective?
Salicylic acid seems to work at reasonably high pHs, but if you’d like to make sure, I’d recommend that you adjust the pH right before you use it to avoid destabilising the formula. It doesn’t need to be kept at low pH.
Could you please explain why the pH is raised in products containing glycolic acid if the raise in pH is counterproductive and makes the glycolic acid less effective?
Why not use less glycolic acid and not raise pH for the same(?) effect?
Some of the ionised glycolic acid will turn back into free acid over time, so it gives a slow release effect.
I disagree with Michelle on this one. (We are both chemists. I am a biochemist and spectroscopist with cosmetic chemistry experience.) Acids sold to skincare professionals do not typically have the pH raised. The reason OTC products are buffered is to make them “safer” for home use (espcially with respect to sun sensitivity). The FDA guides manufacturers on the pH of OTC AHAs via a 2005 document. An AHA is considered in compliance when ” The final product has a pH of 3.5 or greater.” Skincare companies compensate for the FDA pH guideline by using more acid. (There are similar gudelines or regulations in Europe, Canada, etc.) I make my own acids (AHAs & salicylic) or buy them from professional websites and they are not buffered at all. The pH is generally just under 2.0 when these acids are not adjusted to a higher pH. OTC salicyclic acid is not strong enough for my pores!
I can’t work out which part you disagree with?
This is awesome. Thanks so much Michelle. May I ask, does this work with PHAs? I would be interested to know if it’s possible to calculate how much free acid is present in a formulation of 5% gluconic acid at pH 4.5.
It should work, but I think PHAs don’t need to be in free acid form to work…
How about betaine salicylate?
Hi, I wonder if this brilliant calculator is still available? I used to be able to access it, but now all I get is network errors (when there aren’t any), and “item not available”.
It should work now! I think I fixed whatever was going on…
It worked for me before, but now it downloads as a pdf instead of Excel :/
Argh how annoying! I honestly have no idea what Sheets is doing, it’s like every time I open the sheet something new has happened…
Sadly, the link still opens as a read only html file. There is no way to copy it either, because it won’t open in Sheets at all. Only the old free acid calculator can be found in Google Sheets, but maybe it would work if you copy pasted the new one there?
It’s now working again! Thank you! xxx
Thank you! it was really helpful. I have a doubt by using AHA’s and BHA’s the pH value is decreasing so am loosing the thickness of the cream. How can I maintain both.
Use another thickening agent that is not pH sensitive. Xanthan gum is a good alternative.
Hi! I am working ona project and this site came in very handy!
When skincare products state a percentage of concentration, what is meant? 10% of the mass of 10% of the volume?
Either one – density is approximated to be 1 g/mL for aqueous products (the concentration generally isn’t precise enough for density to make much of a difference, except for drug products which are usually w/w).
Serious & important question…
I have solutions of 100% TCA (mind mindbogglingly dangerous scarred for life stuff) & also 70% glycolic acid.
I want to dilute them both to much lower percentages.
I don’t know the pH for either of them.
It is true that you can simply use the formula C1 X V1 = C2 X V2 ???
All the “bro science” & dodgy forums say that you can use this formula. So you don’t need to use a formula which includes things like the pKa or pH of the orginal solution….
Can it really be this easy Michelle?
c1V1 = c2V2 works for finding out the new percentage of total acid, but it doesn’t work for % free acid since the equilibrium shifts upon dilution.
Thanks so much for replying MIchelle (I surprised to get a reply from a minor celebrity!)
So what would be the formula for calculating the % of free acid after the dilution of the 100% TCA or 70% glycolic acid?
I assume this formula would include perhaps the pKa & the pH
I can buy pH testing strips so can get that information if it’s needed.
Maybe each acid has it’s own unique formula :/
For that sort of calculation, you’d change the concentration of the acid at the start and perform the calculation normally e.g. if you have 100% TCA and dilute it 10 times, put it into the calculator as 10% TCA, then measure the pH of the solution and put that in.