Warning: Very nerdy content ahead!
If you’ve been following sunscreen discourse (apparently this is a thing now) you’ll know that mineral sunscreens mostly work by absorbing UV and only scattering a small amount, in the region of 4-5% across the range of UV. This was quantified in a pretty commonly cited 2016 paper by Curtis Cole, Thomas Shyr and Hao Ou-Yang, “Metal oxide sunscreens protect skin by absorption, not by reflection or scattering”.
But in their experimental setup, they took measurements of pure zinc oxide and titanium dioxide blended into a petrolatum base. What about real sunscreens? Actual sunscreens are usually far more complex emulsions with many more ingredients. Will this make a difference?
Related post: How Do Sunscreens Work? The Science (with video)
One sunny Saturday morning I came across a whitepaper from PerkinElmer, a company that specialises in analytical instruments (amongst other things) titled “Particle Characterization of UV Blocking Sunscreens and Cosmetics Using UV/Visible Spectroscopy”. They present a bunch of graphs for what happens when they shine UV onto 7 sunscreens: 4 organic sunscreens (SPF 15, 30, 50 and 100) and 3 inorganic sunscreens containing metal oxide nanoparticles.
Interestingly, the authors of the study assume that mineral sunscreens only scatter and don’t absorb UV. They attribute all of the (rather substantial) UV absorbance to the “inactives” in the sunscreen base, which seems like a bit of an oversight – sunscreen bases aren’t that dissimilar to moisturiser bases, so if that were true a lot of moisturisers would give very good SPF protection! But the information in the paper still lets us work out what’s going on, and apparently I have nothing better to do on a Saturday morning, so I decided to try to milk the data I wanted out of a paper that was meant to be for something else entirely…
UV and sunscreen
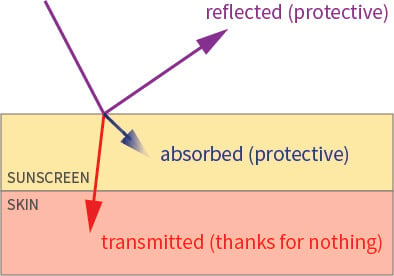
The UV that hits a sunscreen can be either absorbed, reflected (including backscattering) or transmitted (including forward scattering). The UV that’s transmitted goes into your skin, while the UV that’s absorbed and reflected won’t – that’s the UV your skin is protected against:
We’re trying to work out how much UV is reflected, as a percentage of the total UV the sunscreen protects against (absorbed and reflected). That means we need the UV(reflected) and the UV(absorbed), which we can then plug into the formula below:
\%\text{ protection by reflection} = \dfrac{\text{UV}_{\text{reflected}}}{\text{UV}_{\text{reflected}}+\text{UV}_{\text{absorbed}}}\times 100
(Apparently WordPress has LaTeX functionality that I just never knew about…)
These can both be extracted from the study, but it’s indirect and a bit fiddly. (Skip the next section if you don’t really care about the nitty gritty details.)
Extracting the data
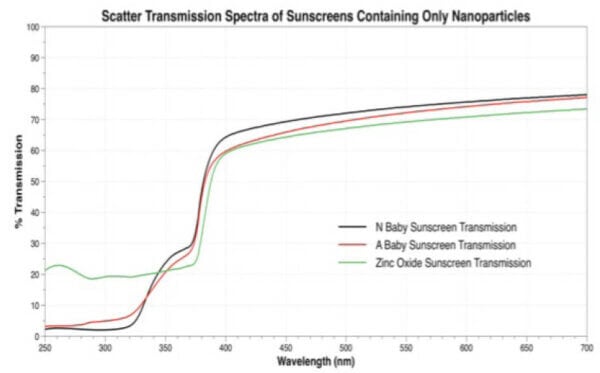
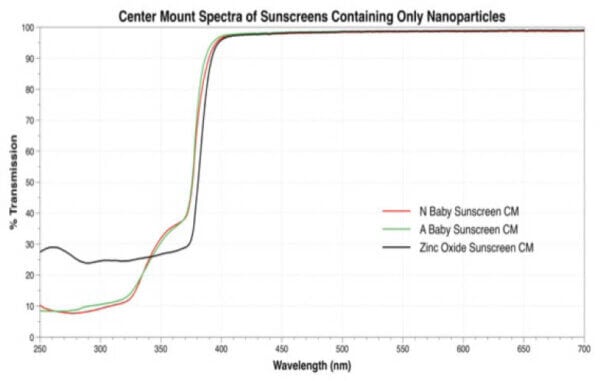
The authors of the study smeared sunscreen onto tape or plates, and then measured the UV transmitted (“transmitted”) as well as the total UV reflected and transmitted (“center mount”), as a percentage of the total UV (absorbed + transmitted + reflected).
To get the UV reflected, we need to measure the area between the “center mount” and “transmitted” curves.
To get the UV absorbed, we need to measure the area above the “center mount” curve.
Annoyingly the two curves for the one sunscreen are on separate graphs. So I threw them into a vector drawing program and lined up the axes of the two graphs, then traced the curves manually as best I could. To integrate them, I counted the pixels in each region. I repeated this process for all of the sunscreens, except for the SPF 15 sunscreen because everything took way too long and I got bored, and do any of us really care that much about SPF 15 these days? Yeah, didn’t think so…
For example, here are the graphs in the paper for the nanoparticle sunscreens (yes, the colours assigned to each sunscreen change between the two graphs just to annoy us):
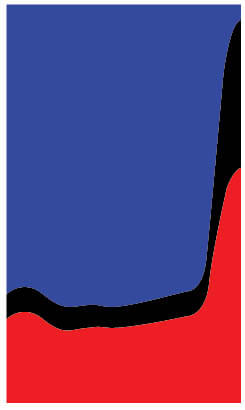
I overlaid and traced the curves for “Zinc Oxide Sunscreen CM” for the UV region (250-400 nm) to get this:
UV reflected is the area between the “center mount” and “transmitted” curves (black), UV absorbed is the area above the “center mount” curve (blue).
One big limitation here is that there’s some wiggle room when tracing the curves (they’re relatively thick lines), so the calculated values might be a bit more precise than they ought to be – treat them as ballpark figures.
How much UV is reflected?
Here’s the UV reflected as a percentage of the total UV protection for the different sunscreens:
- Organic SPF 30 = 7.5%
- Organic SPF 50 = 6.7%
- Organic SPF 100 = 4.2%
- N Baby (SPF 60, 4.7% ZnO + 4.9% TiO2) = 12.4%
- A Baby (SPF 50, 6.8% ZnO + 8.1% TiO2) = 13.0%
- Zinc Oxide (SPF 30, 20% ZnO) = 12.4%
It’s interesting to see that yes, there is actually some reflection from the organic sunscreens which we wouldn’t expect from the active ingredients. The reflection also decreases as a percentage of the total UV protection, so it looks like it doesn’t have to do with the actives, but with something that remains constant as the active ingredients increase and absorb more UV.
My guess would be that it’s from SPF boosters (e.g. refracting microspheres) in the base of the sunscreen that I’ve discussed before, or scattering from emulsion droplets. Sunscreens contain microscopic droplets which usually scatter some light – that’s why emulsion products like creams and lotions usually look opaque and white. In a real product, the emulsion droplets should coalesce and spread out on skin after you apply, but for this experiment, they used a lot more than you’d normally apply and just smeared it onto tape (it was 0.4 mm at the thickest part, while 2 mg/cm2 roughly converts to 0.02 mm thickness).
Assuming that the reflection from the base is roughly the same for organic and inorganic sunscreens with the same SPF, we can subtract the organic sunscreen reflectances from the inorganic ones to estimate the contribution from the inorganic mineral filters only (I just did a linear interpolation between SPF 50 and 100 for the SPF 60 sunscreen because I’m lazy):
- N Baby (SPF 60, 4.7% ZnO + 4.9% TiO2) = 12.4% – 6.2% = 6.2%
- A Baby (SPF 50, 6.8% ZnO + 8.1% TiO2) = 13.0% – 6.7% = 6.3%
- Zinc Oxide (SPF 30, 20% ZnO) = 12.4% – 7.5% = 4.9%
So we end up in the same region of values as the 4-5% from the Cole paper, which I found very satisfying!
References
Cole C, Shyr T, Ou-Yang H. Metal oxide sunscreens protect skin by absorption, not by reflection or scattering. Photodermatol Photoimmunol Photomed. 2016;32(1):5-10. DOI:10.1111/phpp.12214






Who doesn’t love a Saturday of pixel counting? 😉 Thanks for doing the legwork, this was really interesting!
Thanks! To be honest by “I counted them” what I really mean is “I googled for ages to work out how to do it with a program” – trying to understand the data (the paper is actually about something completely different) and tracing the curves was what really took forever!
The worry will be a better place if there are more smart and hard working people like you Michelle 👏 So this is what smart people do on a lazy Saturday afternoon LOL. Interesting read
So, in summary, just pick a sunscreen and wear it and reapply it, correct? 🙂
Yes! It’s really easy to overthink sunscreen and add in unnecessary myths and misconceptions…
Not ‘nerdy’ – 𝘵𝘦𝘤𝘩𝘯𝘪𝘤𝘢𝘭! (& great work / article! ; ) I dislike the pile of derogatory terms & labels, mostly usa invented, for anyone ‘different’ to the labeller. I guess using them positively, as Gay people did with ‘queer’, etc can change it but I wish people -(& I don’t mean the writer, I mean society)- would just stop trying to divide humanity with daft labels..
I think nerdy and geeky are used as self-identifying non-derogatory labels these days 🙂
Oh wow, what a way to spend a sunny Saturday morning. Thank you for doing that so I don’t have to!
This is a great post! You are an amazing skincare detective 🙂
Will non-nano zinc work better or will the result be similar?
Natural brands use non-nano. Thank you
Non-nano generally doesn’t work better than nano and coated – the synthetic coatings help the zinc stay spread out, and smaller particles stay spread out better as well.
Thank you, this is very interesting.